Axinix 5 Mg (Axitinib)
Related products
Product Description
Axinix 5 mg (Axitinib) is a kinase inhibitor indicated for the treatment of advanced renal cell carcinoma after failure of one prior systemic therapy.
Product Features:
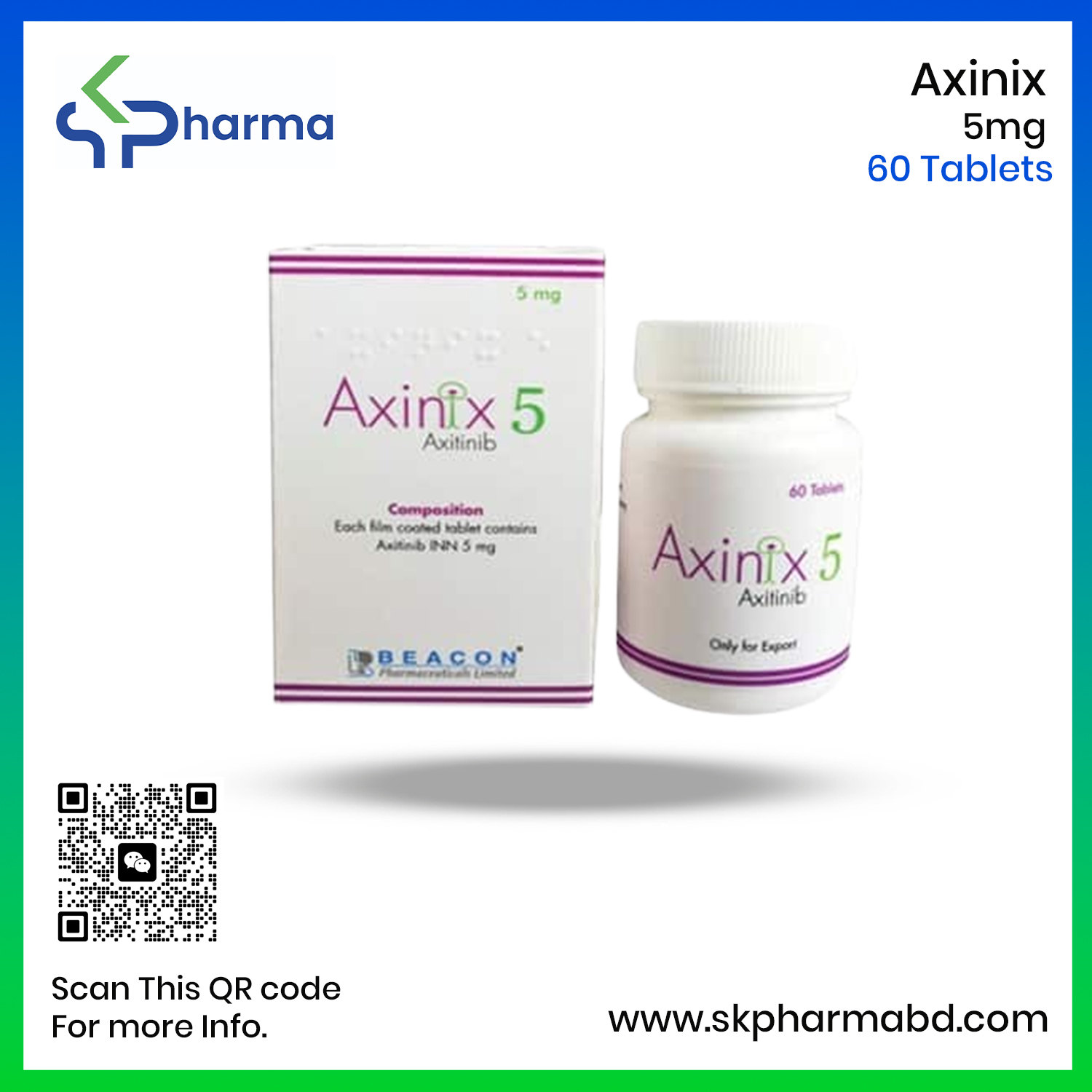
Product Name: Axinix
Generic Name: Axitinib
Formulation: Tablet
Available Pack Size: 180’s Pot & 60’s Pot
Available Strengths: 1 mg & 5 mg
Categories: Anti-viral, Biotech Products, Drugs to treat Hepatitis
Manufacturer: Beacon Pharmaceuticals Ltd.
COMPOSITION
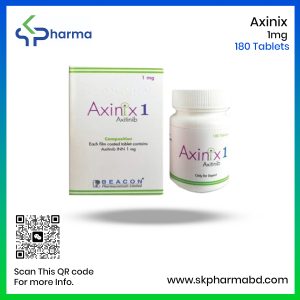
Axinix 1 Tablet: Each film-coated tablet contains Axitinib INN 1 mg.
Axinix 5 Tablet: Each film-coated tablet contains Axitinib INN 5 mg.
CLINICAL PHARMACOLOGY
Mechanism of Action
Axitinib has been shown to inhibit receptor tyrosine kinases including vascular endothelial growth factor receptors (VEGFR)-1, VEGFR-2, and VEGFR-3 at therapeutic plasma concentrations. These receptors are implicated in pathologic angiogenesis, tumor growth, and cancer progression. VEGF-mediated endothelial cell proliferation and survival were inhibited by Axitinib in vitro and in mouse models. Axitinib was shown to inhibit tumor growth and phosphorylation of VEGFR-2 in tumor xenograft mouse models.
INDICATION
Axitinib is indicated for the treatment of advanced Renal Cell Carcinoma (RCC) after the failure of one prior systemic therapy.
DOSAGE AND ADMINISTRATION
Recommended Dosing
The recommended starting oral dose of Axitinib is 5 mg twice daily. Administer Axitinib doses approximately 12 hours apart with or without food. Axitinib should be swallowed whole with a glass of water.
If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time.
CONTRAINDICATIONS
None
ADVERSE REACTIONS
The most common (>_20%) adverse reactions are diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar-plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia and constipation.
DRUG INTERACTIONS
In vitro data indicate that Axitinib is metabolized primarily by CYP3A4/5 and, to a lesser extent, CYP1A2, CYP2C19, and uridine diphosphate-glucuronosyltransferase (UGT) 1A1.
CYP3A4/5 Inhibitors
Co-administration of Ketoconazole, a strong inhibitor of CYP3A4/5, increased the plasma exposure of Axitinib in healthy volunteers. Co-administration of Axitinib with strong CYP3A4/5 inhibitors should be avoided. Grapefruit or grapefruit juice may also increase Axitinib plasma concentrations and should be avoided. Selection of concomitant medication with no or minimal CYP3A4/5 inhibition potential is recommended. If a strong CYP3A4/5 inhibitor must be coadministered, the Axitinib dose should be reduced.
CYP3A4/5 Inducers
Co-administration of Rifampin, a strong inducer of CYP3A4/5, reduced the plasma exposure of Axitinib in healthy volunteers. Co-administration of Axitinib with strong CYP3A4/5 inducers (e.g., Rifampin, Dexamethasone, Phenytoin, Carbamazepine, Rifabutin, Rifapentine, Phenobarbital, and St. John’s wort) should be avoided. Selection of concomitant medication with no or minimal CYP3A4/5 induction potential is recommended. Moderate CYP3A4/5 inducers (e.g., Bosentan, Efavirenz, Etravirine, Modafinil, and Nafcillin) may also reduce the plasma exposure of Axitinib and should be avoided if possible.
PHARMACEUTICAL INFORMATION
Storage Conditions
Store in a cool and dry place, away from light. Keep out of the reach of children.
Presentation & Packaging
Axinix 1 Tablet: Each commercial box contains 180 tablets in a bottle.
Axinix 5 Tablet: Each commercial box contains 60 tablets in a bottle.