Related products
Product Description
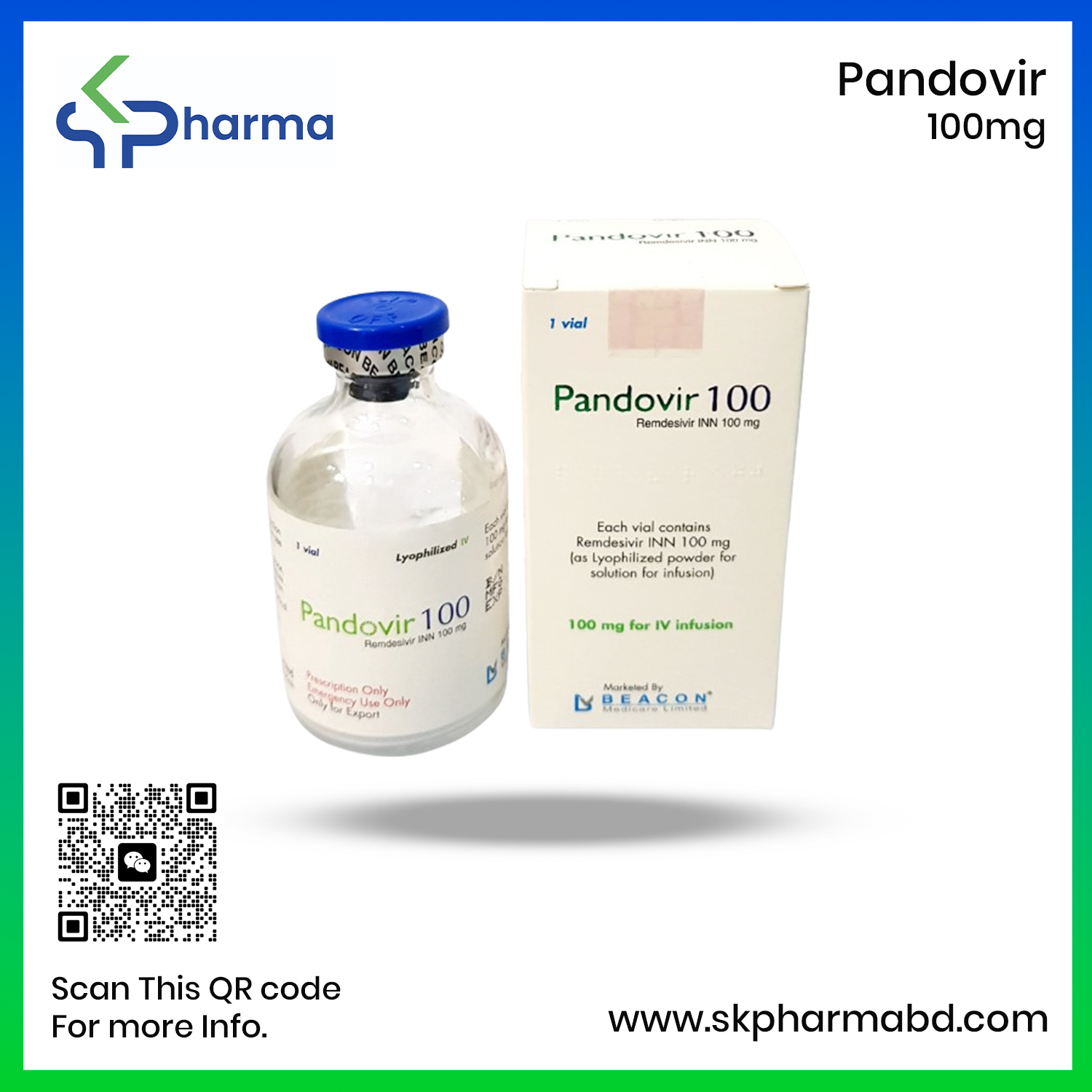
Beacon Pharmaceuticals Limited (BPL) has launched Pandovir 100, a broad-spectrum antiviral medicine, for the treatment of severe Covid-19 cases under the generic of Remdesivir.
Directorate General of Drug Administration (DGDA) considered it as an Emergency Use Authorization (EUA) for the patients who are hospitalized with probable or laboratory-confirmed case of SARS-CoV-2.
The medicine will be available in the market in a single strength –100mg –injection with a new packaging availing the brand name Pandovir,that will open a new horizon for treating the severely ill Covid-19 patients.
The Packaging includes 2 vial (1 vial of Remdisivir 100 mg Lyophilized powder & 1 vial of 20 mL WFI) and a leaflet.