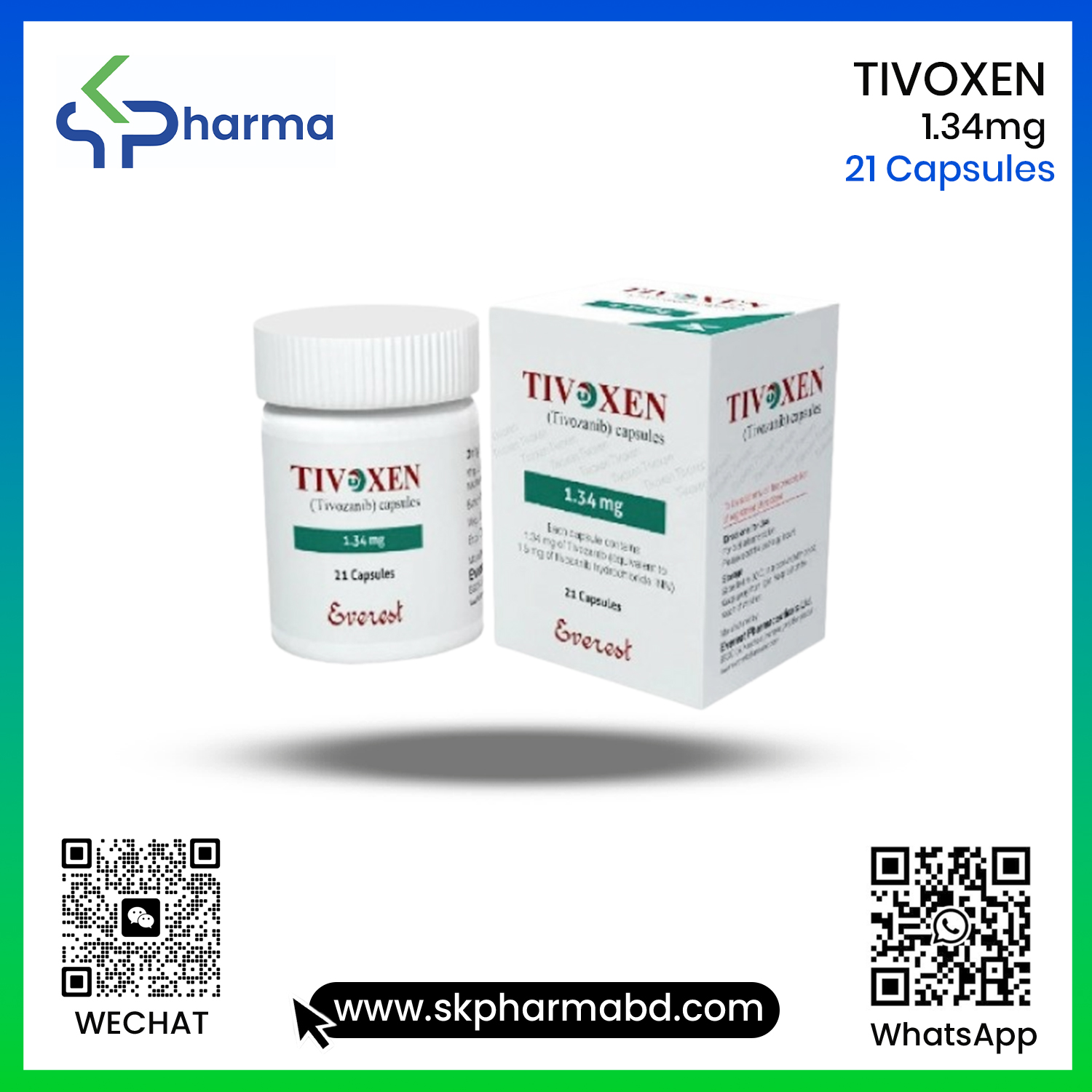
TIVOXEN 1.34 mg (Tivozanib )
Related products
Product Description
COMPOSITION: Tivoxen capsule: Each capsule contains 1.34mgof Tivozanib equivalent to 1.5 mg of tivozanib hydrochloride INN. PHARMACOLOGY Mechanism of Action : Tivozanib is a tvrosine kinase inhibitorIn vitro cellular kinase assays demonstrated that Tivozanib inhibits phosphorylation of vascular endothelial growth factor receptor(VEGFR)-1VEGFR-2 and VEGFR-3 and inhibits other kinases including C-kit and PDGFR at clinically relevant concentrations. In tumor xenograft models in mice and ratsTivozanib inhibited angiogenesisvascular permeability, and tumor growth of various tumor cell types including human renal cell carcinoma.
INDICATIONS:
Tivozanib is indicated for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma(RCC)following two or more prior systemic therapies.
DOSAGE AND ADMINISTRATION :
Recommended Dosing
The recommended dosage of Tivozanib is 1.34 mg taken orally once daily for 21 days on treatment followed by7 days off treatment for a 28-day cycle.
Continue treatment until disease progression oruntil unacceptable toxicity occurs
Take Tivozanib with or without food.Swallow theTivozanib capsule whole with a glass of waterDo not openthe capsule
If a dose is missedthe next dose should be taken at the next scheduled timeDo not take two doses at the same time.
Dose Modifications for Adverse Reactions:
Initiate medical management for diarrheanauseaor vomiting prior to dose interruption or reduction.
If dose modifications are reauired for adverse reactions reduce the dosage of Tivozanib to0.89mg for21 days on treatment followed by 7 days off treatment for a28-day cycle.
CONTRAINDICATIONS :
None.
WARNINGS AND PRECAUTIONS:
Hypertension and Hypertensive Crisis:-
Tivozanib can cause severe hypertension and hypertensive crisis.Hypertension occurred in 45% ofpatients treated with Tivozanib. with 22% ofthe events Grade 3Median time to onset of hypertension was 2weeks(range:0-192 weeks)
Cardiac Failure:
Tivozanib can cause serioussometimes fatalcardiac failure.Cardiac failure in Tivozanibtreated patients occurred in 1.6%. with 1% of eventsGrade 3and 0.6% events were fatal
Periodically monitor patients for symptoms of cardiac failure throughout treatment with Tivozanib
Discontinue Tivozanib in patients who develop any severe or life-threatening arterial thromboembolic event.
Venous Thromboembolic Events:
Closely monitor patients who are at risk foror who have a history of these events during treatment with Tivozanib Discontinue Tivozanib in patients who develop any severe or life-threatening venous thromboembolic event.
Hemorrhagic Events:
Discontinue Tivozanib in patients who develop severe or life-threatening hemorrhagic events.
Proteinuria:
Monitor patients for proteinuria before initiation ofand periodically throughout, treatment with Tivozanib
Thyroid Dysfunction:
Treat hypothyroidism and hyperthyroidism to maintain euthyroid state before and during treatment with Tivozanib
Risk of Impaired Wound Healing:
Withhold Tivozanib for at least 24 davs prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Tivozanib after resolution of wound healing complications has not been established
Reversible Posterior Leukoencephalopathy Syndrome(RPLS) Perform an evaluation for RPLS in anypatient presenting with seizures,headachesvisual disturbances confusion, or altered mental function.Discontinue Tivozanib in patients who develop RPLS
Embryo-Fetal Toxicity:
Advise females of reproductive potential to use effective contraception during treatment with Tivozanib and for one month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Tivozanib and for one month after the last dose
SIDE EFFECTS:
The following clinically significant adverse reactions are also described elsewhere in the labeling·Hypertension and Hypertensive Crisis Cardiac Failure
·Cardiac Ischemia and Arterial Thromboembolic Events oVenous Thromboembolic Events·Hemorrhagic Events·Proteinuria
·Thyroid Dysfunction
·Risk of Impaired Wound Healing
·Reversible Posterior Leukoencephalopathy Syndrome(RPLS). OVERDOSE
Overdosage with Tivozanib can cause severe hypertension and hypertensive crisis that may result in death.
There is no specific treatment or antidote for Tivozanib overdose.
In cases of suspected overdosewithhold Tivozanib closely monitor patients for hypertension andhypertensive crisis and other potential adversereactionsImmediately manage signs or symptoms of hypertension and provide other supportive care as clinically indicated.
STORAGECONDITION
Store below 30°Cin a cool and dry place.Keep awayfrom light. Keep out of the reach of children.
HOW SUPPLIED
Tivoxen capsule: Each HDPE container contains 21 capsules(each capsule contains1.34mgTivozanib)a silica gel desiccant and polyester coil with a child-resistant closure.